FDA 2541a 2012-2026 free printable template
Show details
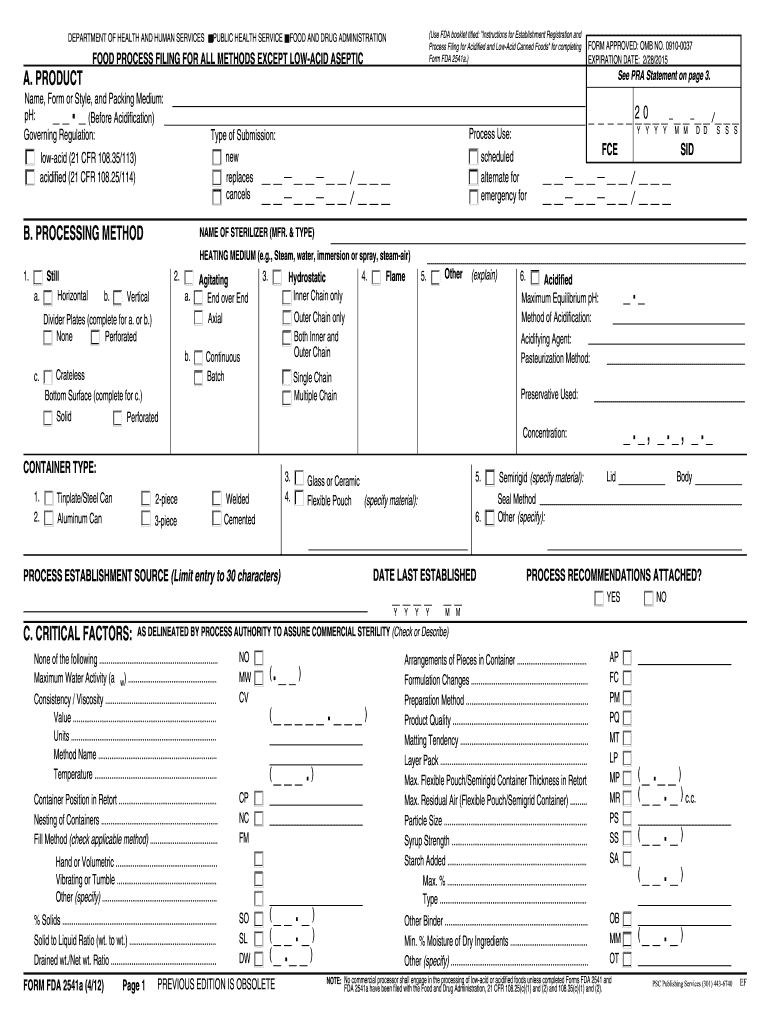
Vibrating or Tumble. FORM FDA 2541a 4/12 OB OT NOTE No commercial processor shall engage in the processing of low-acid or acidified foods unless completed Forms FDA 2541 and FDA 2541a have been filed with the Food and Drug Administration 21 CFR 108. DEPARTMENT OF HEALTH AND HUMAN SERVICES A. PRODUCT PUBLIC HEALTH SERVICE Use FDA booklet titled Instructions for Establishment Registration and Process Filing for Acidified and Low-Acid Canned Foods for completing Form FDA 2541a. FOOD AND DRUG...
pdfFiller is not affiliated with any government organization
Get, Create, Make and Sign ucla site pdffiller com site blog pdffiller com form

Edit your fda 2541 form online
Type text, complete fillable fields, insert images, highlight or blackout data for discretion, add comments, and more.

Add your legally-binding signature
Draw or type your signature, upload a signature image, or capture it with your digital camera.

Share your form instantly
Email, fax, or share your fda form 2541 form via URL. You can also download, print, or export forms to your preferred cloud storage service.
Editing FDA 2541a online
Use the instructions below to start using our professional PDF editor:
1
Set up an account. If you are a new user, click Start Free Trial and establish a profile.
2
Prepare a file. Use the Add New button. Then upload your file to the system from your device, importing it from internal mail, the cloud, or by adding its URL.
3
Edit FDA 2541a. Rearrange and rotate pages, insert new and alter existing texts, add new objects, and take advantage of other helpful tools. Click Done to apply changes and return to your Dashboard. Go to the Documents tab to access merging, splitting, locking, or unlocking functions.
4
Get your file. Select your file from the documents list and pick your export method. You may save it as a PDF, email it, or upload it to the cloud.
Dealing with documents is simple using pdfFiller.
Uncompromising security for your PDF editing and eSignature needs
Your private information is safe with pdfFiller. We employ end-to-end encryption, secure cloud storage, and advanced access control to protect your documents and maintain regulatory compliance.
How to fill out FDA 2541a

How to fill out FDA 2541a
01
Obtain the FDA Form 2541a from the FDA website or relevant authority.
02
Fill in the applicant information, including name, address, and contact details.
03
Provide details about the product in question, such as product name, type, and intended use.
04
Complete the section regarding the manufacturing process, including facilities and equipment used.
05
Include any relevant safety data, studies, or evidence required by the FDA.
06
Review the form for completeness and accuracy.
07
Sign and date the form before submission.
Who needs FDA 2541a?
01
Manufacturers seeking FDA approval for specific products.
02
Companies requiring regulatory compliance for new or existing products.
03
Individuals or organizations conducting research that involves FDA-regulated products.
Fill
form
: Try Risk Free






People Also Ask about
Are the FDA regulations a law?
FDA regulations are also federal laws, but they are not part of the FD&C Act. FDA regulations can be found in Title 21 of the Code of Federal Regulations (CFR). FDA follows the procedures required by its "Good Guidance Practice" regulation to issue FDA guidance.
What items does the FDA regulate?
The FDA is responsible for protecting public health by regulating human drugs and biological products, animal drugs, medical devices, tobacco products, food (including animal food), cosmetics, and electronic products that emit radiation.
Which of the following does the FDA not regulate?
The FDA does not regulate the practice of medicine, medical services, the price or availability of medical products and whether they are reimbursed by health insurance or Medicare.
What does the FDA regulate ?
The Food and Drug Administration is responsible for protecting the public health by ensuring the safety, efficacy, and security of human and veterinary drugs, biological products, and medical devices; and by ensuring the safety of our nation's food supply, cosmetics, and products that emit radiation.
What 4 products are regulated by the FDA?
Products We Regulate Food. Drugs. Medical Devices. Radiation-Emitting Products. Vaccines, Blood, and Biologics. Animal and Veterinary. Cosmetics. Tobacco Products.
What does the FDA regulate list?
The FDA regulates a wide range of products, including foods (except for aspects of some meat, poultry and egg products, which are regulated by the U.S. Department of Agriculture); human and veterinary drugs; vaccines and other biological products; medical devices intended for human use; radiation-emitting electronic
For pdfFiller’s FAQs
Below is a list of the most common customer questions. If you can’t find an answer to your question, please don’t hesitate to reach out to us.
How can I send FDA 2541a to be eSigned by others?
FDA 2541a is ready when you're ready to send it out. With pdfFiller, you can send it out securely and get signatures in just a few clicks. PDFs can be sent to you by email, text message, fax, USPS mail, or notarized on your account. You can do this right from your account. Become a member right now and try it out for yourself!
How do I fill out FDA 2541a using my mobile device?
Use the pdfFiller mobile app to fill out and sign FDA 2541a. Visit our website (https://edit-pdf-ios-android.pdffiller.com/) to learn more about our mobile applications, their features, and how to get started.
How do I complete FDA 2541a on an Android device?
Use the pdfFiller mobile app to complete your FDA 2541a on an Android device. The application makes it possible to perform all needed document management manipulations, like adding, editing, and removing text, signing, annotating, and more. All you need is your smartphone and an internet connection.
What is FDA 2541a?
FDA 2541a is a form used by the U.S. Food and Drug Administration (FDA) for reporting the establishment registration and product listing for certain facilities that manufacture food and feed products.
Who is required to file FDA 2541a?
Facilities that manufacture, process, pack, or hold food for human or animal consumption in the United States are required to file FDA 2541a.
How to fill out FDA 2541a?
To fill out FDA 2541a, facilities must provide accurate information about their establishment, including contact information, types of products manufactured, and any other relevant details as specified in the form instructions.
What is the purpose of FDA 2541a?
The purpose of FDA 2541a is to ensure that the FDA has up-to-date information about facilities that handle food and feed products, aiding in regulatory oversight and public health protection.
What information must be reported on FDA 2541a?
Information that must be reported on FDA 2541a includes the name and address of the facility, contact information, the types of food products produced, and any relevant establishment registration details.
Fill out your FDA 2541a online with pdfFiller!
pdfFiller is an end-to-end solution for managing, creating, and editing documents and forms in the cloud. Save time and hassle by preparing your tax forms online.

FDA 2541a is not the form you're looking for?Search for another form here.
Relevant keywords
Related Forms
If you believe that this page should be taken down, please follow our DMCA take down process
here
.
This form may include fields for payment information. Data entered in these fields is not covered by PCI DSS compliance.
























